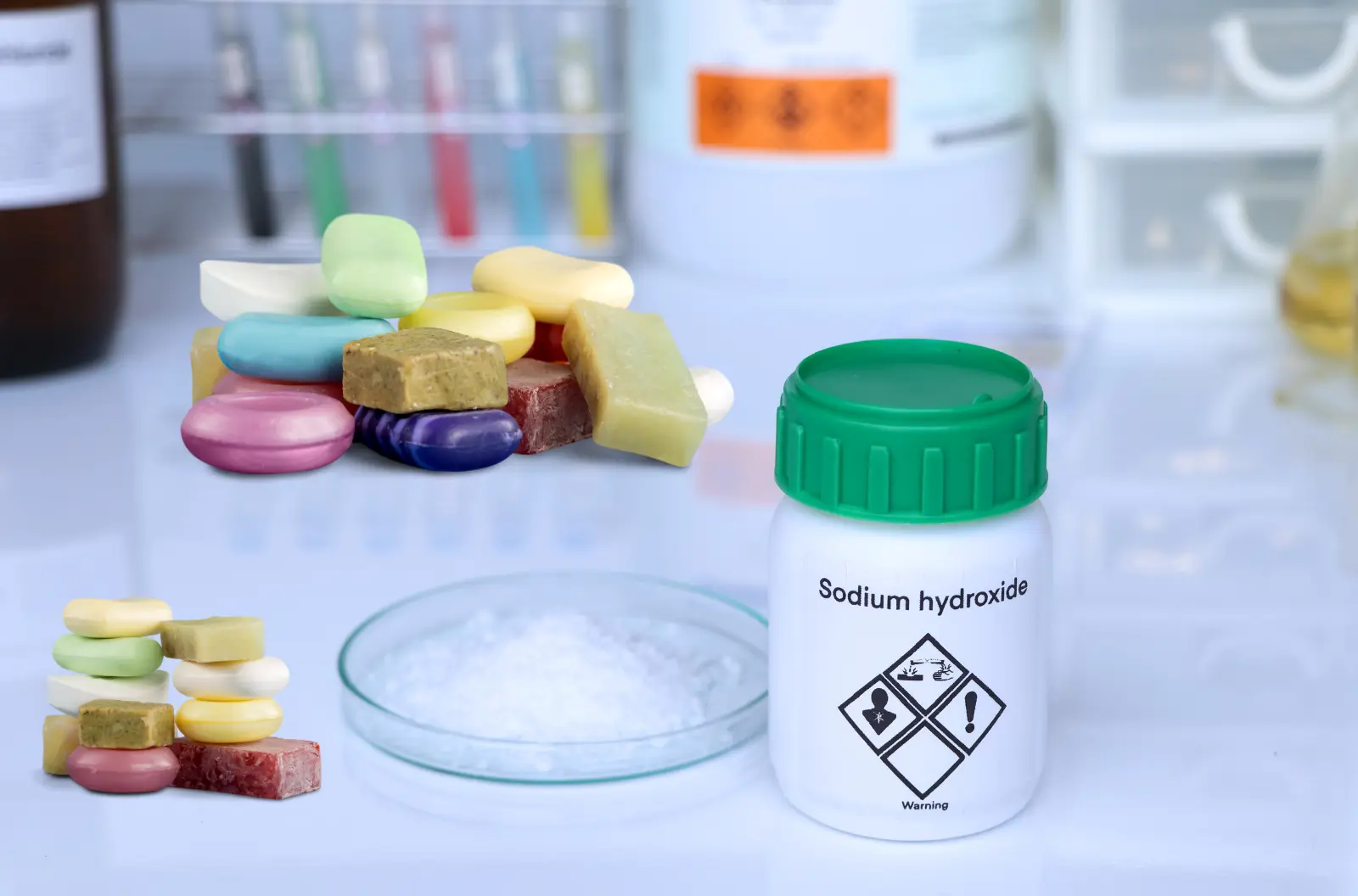
Sodium hydroxide is crucial for the saponification process, which is the chemical reaction that turns fats and oils into soap and glycerin. Without sodium hydroxide, this reaction cannot occur, and soap cannot be made.
When sodium hydroxide reacts with fats or oils, it produces soap that is solid at room temperature. This reaction ensures that the soap has the right consistency and can be molded into bars.
Soap made with sodium hydroxide effectively cleans by breaking down oils and grease on the skin. The alkaline nature of sodium hydroxide allows the soap to emulsify oils, making them easier to wash away.
Soaps made with sodium hydroxide are more stable and have a longer shelf life. The chemical reaction helps to create a product that remains effective and usable over time.
While sodium hydroxide is essential in soap making, it is also highly caustic and must be handled with care. Proper safety measures, such as wearing gloves and goggles, are necessary to prevent injury during the soap-making process.
Sodium hydroxide is a fundamental ingredient in soap making, enabling the saponification process that creates solid, effective, and stable soap. Understanding its role helps in appreciating the science behind soap production.
Sodium Hydroxide, commonly known as lye or caustic soda, is a critical ingredient in soap making. It is used for several important reasons that contribute to the effectiveness and quality of the soap. Here’s a detailed explanation of why sodium hydroxide is used in soap:
The primary reason sodium hydroxide is used in soap is to initiate the saponification process. Saponification is the chemical reaction between a fat or oil and sodium hydroxide, which produces soap and glycerin. This reaction is essential for converting raw fats and oils into a usable soap product.
Sodium hydroxide helps to create solid soap bars. When it reacts with fats or oils, it produces fatty acid salts (the soap) that are solid at room temperature. This solid form is convenient for handling, packaging, and usage.
Using sodium hydroxide in soap making ensures that the soap has a consistent texture and firmness. This consistency is important for both the aesthetic appeal and practical use of the soap. Without sodium hydroxide, the soap mixture would not solidify properly and would remain greasy or oily.
Soaps made with sodium hydroxide have a longer shelf life and are more stable over time. The saponification process produces a product that resists spoiling and maintains its effectiveness, making it suitable for long-term use and storage.
Sodium hydroxide allows soap makers to create a wide variety of soap types and formulations. By adjusting the amounts and types of fats or oils used, as well as the concentration of sodium hydroxide, different textures, hardness levels, and cleansing properties can be achieved. This versatility is valuable for both commercial and artisanal soap production.
While sodium hydroxide is essential for soap making, it is also a highly caustic substance. Proper safety measures, such as wearing protective gloves, goggles, and clothing, are necessary to prevent burns and injuries during the soap-making process. It is important to handle sodium hydroxide with care and follow safety guidelines.
Sodium hydroxide is indispensable in the soap-making process due to its role in saponification, creating solid soap, enhancing cleaning abilities, improving texture, and providing stability. Its use ensures that the final soap product is effective, long-lasting, and suitable for various applications. Understanding the importance of sodium hydroxide in soap helps appreciate the chemistry and craftsmanship behind high-quality soap production.
The question of whether you can make soap without sodium hydroxide (NaOH), also known as lye or caustic soda, is a common one. Sodium hydroxide is traditionally essential for the saponification process, which converts fats and oils into soap. However, there are alternative methods to create soap-like products without directly using sodium hydroxide. Here’s a detailed explanation:
In traditional soap making, sodium hydroxide is indispensable. The saponification process requires a strong alkali like sodium hydroxide to react with fats and oils, creating soap and glycerin. Without this reaction, genuine soap cannot be formed.
One way to make soap without directly handling sodium hydroxide is by using a pre-made soap base, often referred to as melt and pour soap. These bases have already undergone the saponification process and can be melted, customized with additives like fragrances and colors, and then molded into new shapes. This method allows you to create soap without dealing with sodium hydroxide yourself.
Syndet bars (synthetic detergent bars) are another alternative. These bars do not contain traditional soap but are made from synthetic surfactants. They can be designed to mimic the properties of soap, such as cleansing and lathering, without using sodium hydroxide. Syndet bars are commonly found in commercial cleansing products like beauty bars and body washes.
While not exactly the same as avoiding sodium hydroxide, some soap makers use potassium hydroxide (KOH) instead. Potassium hydroxide is used to make liquid soap or soft soap. Although it’s a different alkali, it still performs the essential function of saponification.
Another option is to use natural cleansers that don’t involve the saponification process. Ingredients like soap nuts, yucca root, or castile soap (which is made using traditional methods but with vegetable oils) can provide cleansing properties without the direct use of sodium hydroxide. These natural alternatives are popular among those looking for a more eco-friendly or chemical-free option.
While traditional soap making requires sodium hydroxide for the saponification process, there are alternative methods to create soap-like products without directly using it. Pre-made soap bases, syndet bars, potassium hydroxide for liquid soap, and natural cleansers offer viable options. Each method has its own benefits and limitations, but they all provide ways to avoid handling sodium hydroxide while still achieving cleansing properties.
Understanding these alternatives allows for greater flexibility in soap making and offers solutions for those who prefer not to use sodium hydroxide directly. Whether through melt and pour techniques, synthetic detergents, or natural ingredients, it’s possible to create effective cleansing products without the need for sodium hydroxide.
Sodium Hydroxide, also known as lye or caustic soda, is essential in traditional soap making due to its role in the saponification process, which converts fats and oils into soap and glycerin. It ensures that soap is solid, effective at cleaning, stable, and long-lasting. While handling sodium hydroxide requires caution due to its caustic nature, it remains a fundamental ingredient for producing high-quality soap.
However, there are alternative methods for creating soap-like products without directly using sodium hydroxide. Pre-made soap bases, syndet bars, potassium hydroxide for liquid soap, and natural cleansers like soap nuts and castile soap offer various ways to achieve similar cleansing properties. These alternatives provide options for those who prefer not to handle sodium hydroxide while still creating effective and versatile cleaning products.