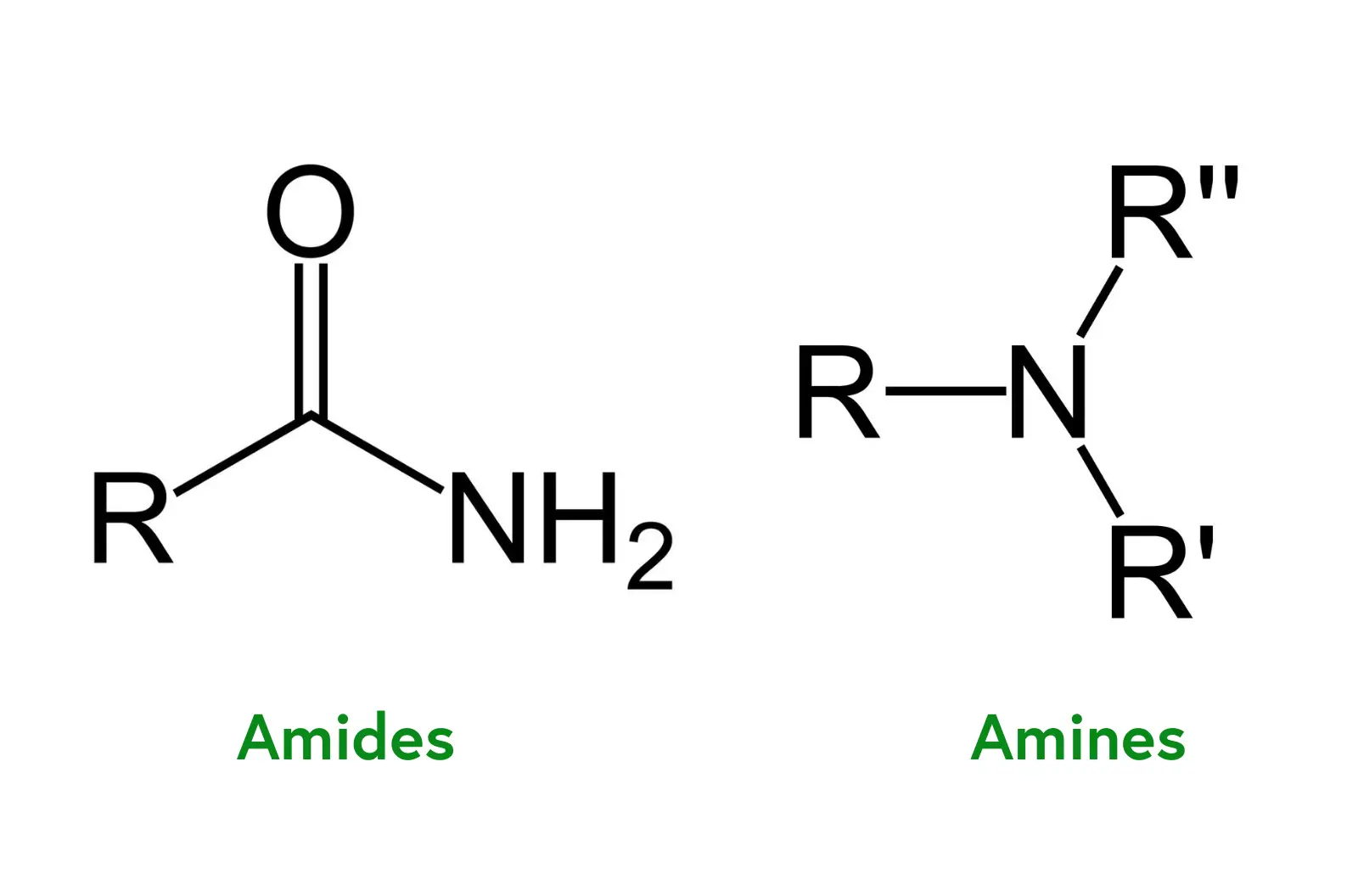
Amides are organic compounds derived from carboxylic acids, where the hydroxyl group (-OH) is replaced by an amino group (-NH₂). This results in a structure characterized by a carbonyl group (C=O) directly bonded to a nitrogen atom. The general formula for amides is R-CO-NH₂, where R represents an organic substituent, which can be an alkyl or aryl group. Amides are found extensively in biological molecules, particularly in proteins, where they form peptide bonds linking amino acids.
The structural differences between amides and amines are pivotal in determining their chemical behaviors and physical properties.
Primary Amides: Have the general structure R-CO-NH₂, with one hydrogen atom and one organic substituent attached to the nitrogen.
Secondary Amides: Have the structure R-CO-NHR, with one hydrogen replaced by an organic group.
Tertiary Amides: Have the structure R-CO-NR₂, with both hydrogen atoms replaced by organic groups.
The carbonyl group in amides creates a planar structure around the C=O bond, which allows for resonance stabilization. This resonance reduces the electron density on the nitrogen, making amides less basic than amines.
Primary Amines: Contain one alkyl or aryl group attached to the nitrogen atom (R-NH₂).
Secondary Amines: Have two alkyl or aryl groups attached (R₂NH).
Tertiary Amines: Feature three alkyl or aryl groups attached (R₃N).
The nitrogen in amines has a lone pair of electrons, which contributes significantly to their reactivity and basicity. The lack of a carbonyl group means there is no resonance stabilization as seen in amides.
Pharmaceuticals: Amides are crucial in drug development due to their stability and ability to form hydrogen bonds, which enhance the effectiveness and longevity of medications. Drugs like acetaminophen and penicillin contain amide groups that contribute to their therapeutic properties.
Materials Science: Amides are the backbone of synthetic polymers such as nylon and Kevlar, which are known for their strength and durability. These materials are used in various applications, from clothing to industrial equipment, providing high-performance characteristics that are essential in demanding environments.
Agriculture: Amides serve as herbicides and pesticides, playing a key role in crop protection. For instance, propanil is used extensively in rice cultivation to control weed growth without damaging the rice plants, enhancing agricultural productivity and sustainability.
Agriculture: Amines are integral to the synthesis of agrochemicals such as glyphosate, a widely used herbicide. These compounds help control weeds and pests, contributing to efficient and sustainable farming practices by improving crop yields and reducing the reliance on manual labor.
Pharmaceuticals: Many pharmaceutical drugs incorporate amine groups to enhance their solubility and reactivity. Compounds such as antihistamines, antidepressants, and antibiotics benefit from the presence of amines, which facilitate their interaction with biological targets and improve their therapeutic efficacy.
Proteins and Peptides: Amides form the backbone of proteins through peptide bonds. These bonds link amino acids together, forming long chains that fold into functional proteins.
Pharmaceutical Industry: Many drugs contain amide groups due to their stability and resistance to metabolic breakdown.
Synthetic Materials: Amides are used in the production of high-strength materials like nylon, Kevlar, and other polyamides.
Neurotransmitters and Hormones: Amines like epinephrine, norepinephrine, and dopamine are critical for transmitting nerve signals and regulating bodily functions.
Chemical Synthesis: Amines are used to create various organic compounds, including pharmaceuticals, dyes, and polymers.
Catalysis: Due to their ability to donate and accept protons, amines are effective catalysts in chemical reactions.
Boiling and Melting Points: Amides generally have higher boiling and melting points compared to amines of similar molecular weight. This is due to the strong hydrogen bonding and the presence of the carbonyl group.
Solubility: Amides are typically soluble in polar solvents like water, especially those with lower molecular weights. The hydrogen bonding with water molecules enhances their solubility.
Stability: Amides are stable compounds, resistant to oxidation and reduction. Their resonance-stabilized structure contributes to this stability.
Boiling and Melting Points: Amines have lower boiling and melting points than amides due to the absence of the carbonyl group. Their boiling points increase with the number of alkyl groups attached to the nitrogen.
Solubility: Amines are also soluble in water, but this solubility decreases as the length of the hydrocarbon chain increases. The lone pair of electrons on the nitrogen allows for hydrogen bonding with water.
Basicity: Amines are basic compounds. The lone pair of electrons on the nitrogen can accept a proton, making them reactive bases and nucleophiles.
Hydrolysis: Amides can be hydrolyzed under acidic or basic conditions to form carboxylic acids and amines or ammonia. This reaction is typically slow and requires a catalyst.
Amidation: Amides can be formed from the reaction of carboxylic acids with amines or ammonia. This process is known as amidation and is a common method of synthesizing amides.
Acylation: Amides participate in acylation reactions, where the acyl group is transferred to another molecule. This reaction is used in organic synthesis to create more complex compounds.
Nucleophilic Substitution: Amines readily undergo nucleophilic substitution reactions due to the lone pair of electrons on the nitrogen. They can react with alkyl halides to form more complex amines.
Protonation: Amines can be protonated by acids to form ammonium salts. This property is used in various chemical processes, including the formation of salts for pharmaceuticals.
Condensation: Amines participate in condensation reactions, forming imines and enamines. These reactions are important in the synthesis of many organic compounds.
Bonding: The presence of a carbonyl group bonded to a nitrogen atom defines amides. This C=O bond is polar and allows for strong hydrogen bonding with other molecules.
Functional Groups: The primary functional group in amides is the -CONH₂ group. This group can participate in various chemical reactions, including hydrogen bonding and nucleophilic substitution.
Bonding: Amines have a nitrogen atom bonded to one or more alkyl or aryl groups. The lone pair of electrons on the nitrogen plays a crucial role in their reactivity.
Functional Groups: The primary functional group in amines is the amino group (-NH₂, -NHR, or -NR₂). This group is basic and can form hydrogen bonds with water and other molecules.